Introduction
Kymera Therapeutics has always been one of my most admired PROTAC companies, as mentioned in previous articles. The STAT6 PROTAC KT-621 project is one of its most valued projects, and its outstanding preclinical data has once again added significant weight to the development of PROTACs in the field of autoimmunity.
KT-621 entered Phase I clinical trials on October 24 of this year, with preliminary results expected to be announced in the first half of 2025.
Undoubtedly, KT-621 is a highly anticipated project. However, to date, Kymera's STAT6 PROTAC project has only one patent lacking implementation examples, which can only be referred to as the platform technology patent WO2024064080A1 (which protects all reported combinations of STAT6 inhibitors and E3 ligase ligands), published on March 28, 2024. This indeed leaves fast-follower companies at a loss.
This patent does provide valuable information. I initially wanted to wait for the compound patent, but now I can no longer afford to wait. Below, based on this 345-page patent, I will unravel and glimpse the general structure of KT-621.
Since PROTAC is a ternary complex bifunctional compound, I will conduct a structural analysis from the three components: E3 ligand, Linker, and warhead.
Due to the length of the text, this article will only analyze the warhead portion, listing the currently disclosed STAT6 inhibitor projects.
Warhead
There is quite a bit of information worth analyzing regarding the warhead. It is very considerate that Kymera has listed all currently disclosed STAT6 inhibitors as warhead ideas in the patent. Therefore, this article will take the opportunity to organize this information (listing the original patent structures, corresponding PROTAC patent structures, and representative compound structures from the original patents) and categorize it into several parts based on the connections between the patents:
1. Common aromatic heterocycles, low reference value
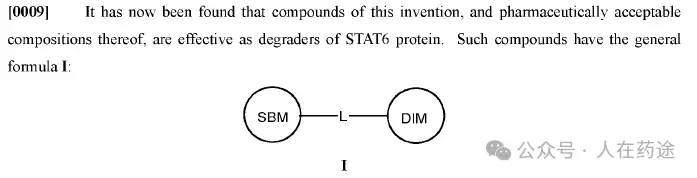
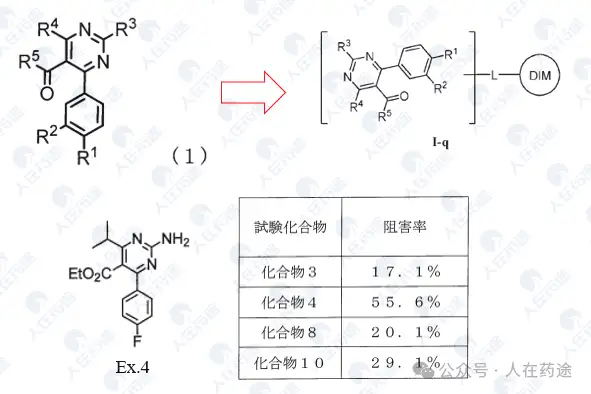
JP1999116481A, Sumitomo Pharmaceuticals
JP1999106340A, Sumitomo Pharmaceuticals
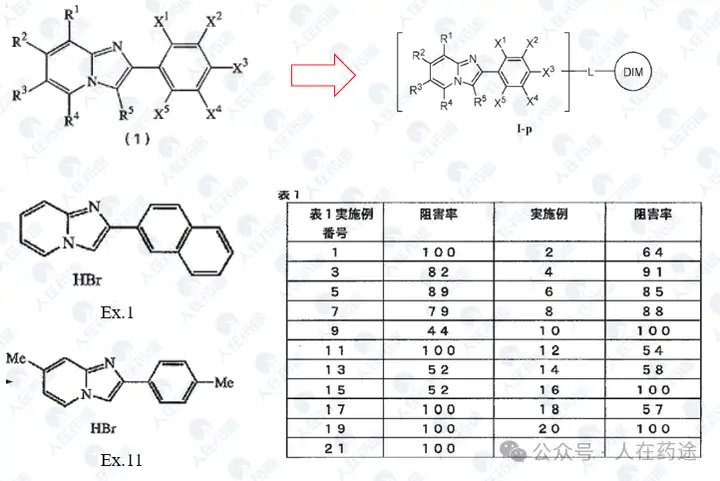
JP1999029475A, Sumitomo Pharmaceuticals
JP2000229959A, Sumitomo Pharmaceuticals
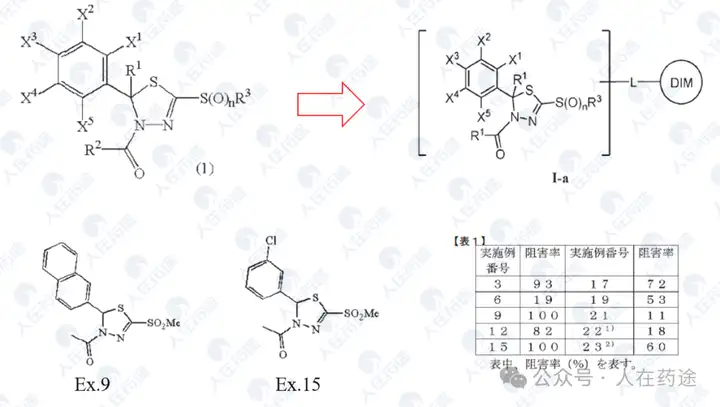
JP2008050319A, Kaken Pharmaceutical Co., Ltd.
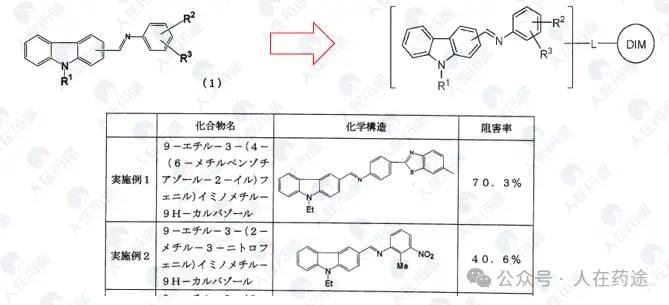
JP2007297307A, Kaken Pharmaceutical Co., Ltd.
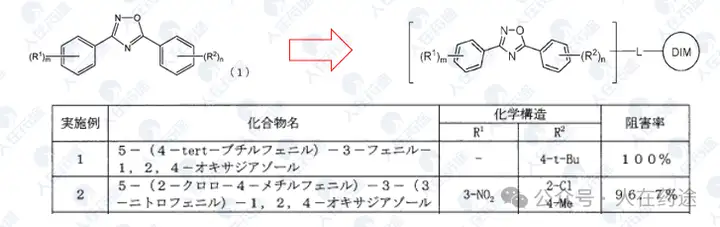
JP2008031107A, Kaken Pharmaceutical Co., Ltd.
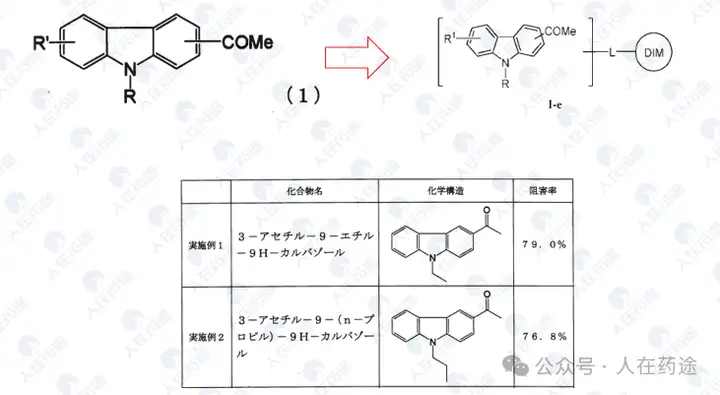
JP2008273852A, Kaken Pharmaceutical Co., Ltd.
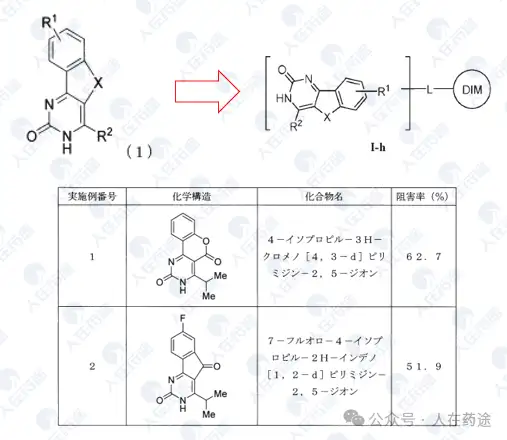
JP2008110935A, Kaken Pharmaceutical Co., Ltd.
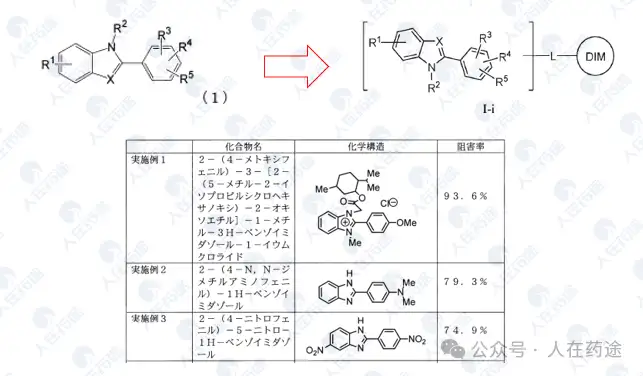
JP2008081460A, Kaken Pharmaceutical Co., Ltd.
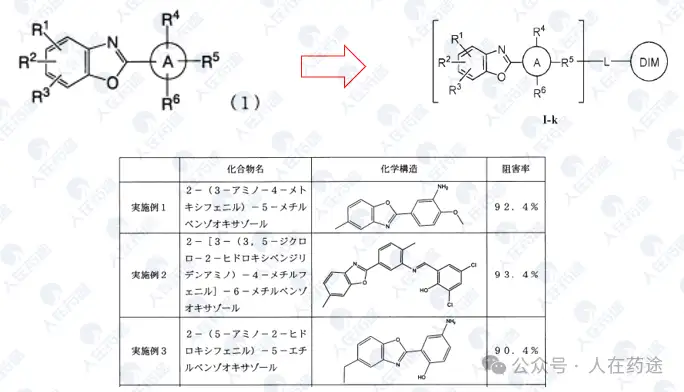
JP2008208103A, Kaken Pharmaceutical Co., Ltd.
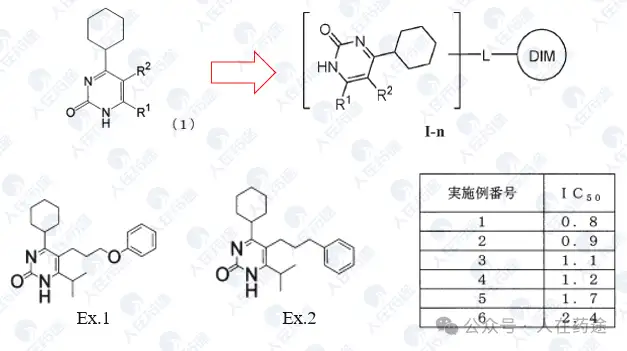
JP2008162978A, Kaken Pharmaceutical Co., Ltd.
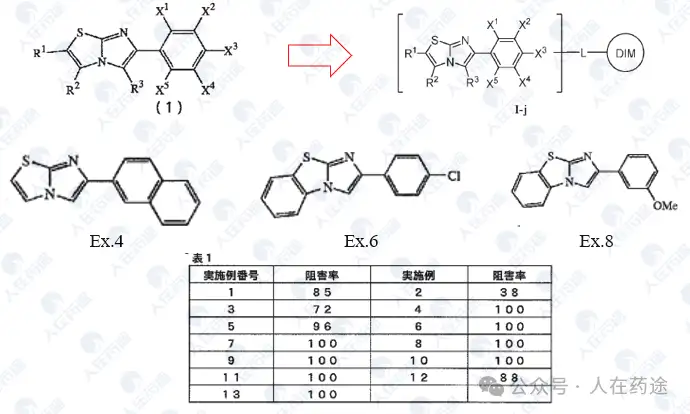
In summary, the above patents are all STAT6 inhibitors discovered by Japanese companies, but most of the patents only report inhibition rates. Judging by the structures, the inhibition activity and selectivity are likely not ideal (these patents have not been authorized, and their legal status is all withdrawn, which can also be proven), and they are unlikely to be true warheads.
2. High-activity STAT6 inhibitors from MNCs
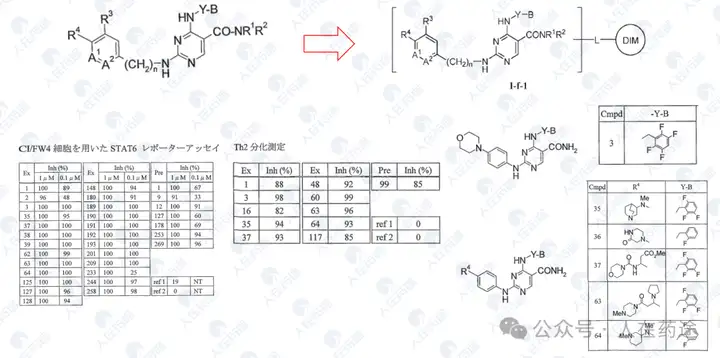
WO2004002964A1, Yamanouchi Pharmaceutical (predecessor of Astellas)
Astellas, Bioorganic & Medicinal Chemistry, 2007, 15(2): 1044-1055.
JP2006241089A, Astellas
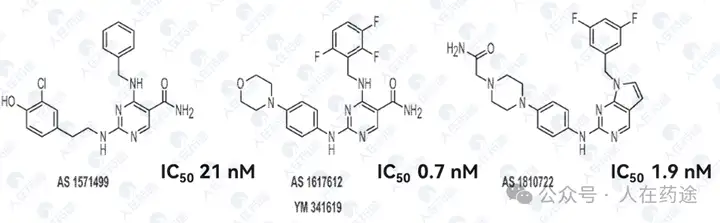
Astellas's representative STAT6 inhibitor (shown below) is the most studied compound in academia. Although it has high STAT6 inhibitory activity, and many literatures indicate good anti-inflammatory activity for this type of compound, Astellas has not conducted further research based on this. It is speculated that there may be defects in druggability or selectivity.
WO2002079165A1, AstraZeneca
The representative compound has good STAT6 inhibitory activity.
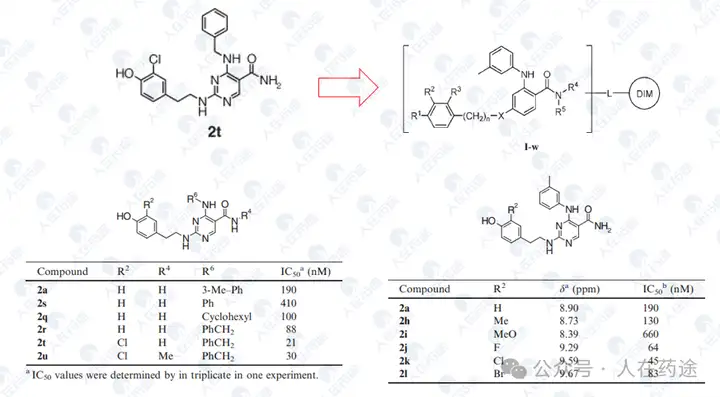
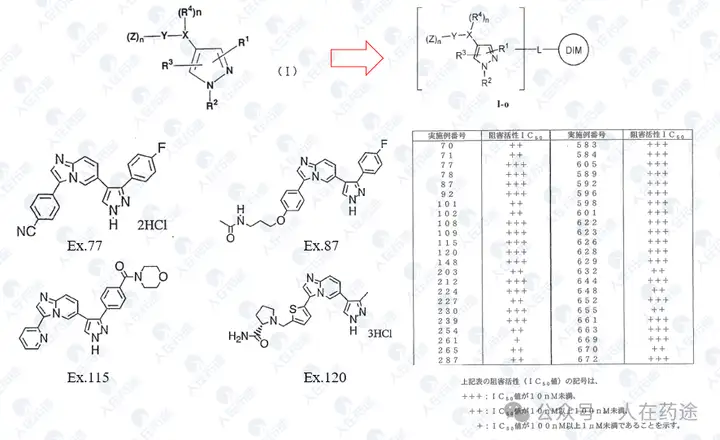
WO2002088107A1, Eisai
Most compounds in the patent exhibit excellent STAT6 inhibitory activity (IC50 < 10 nM), but selectivity is unknown.
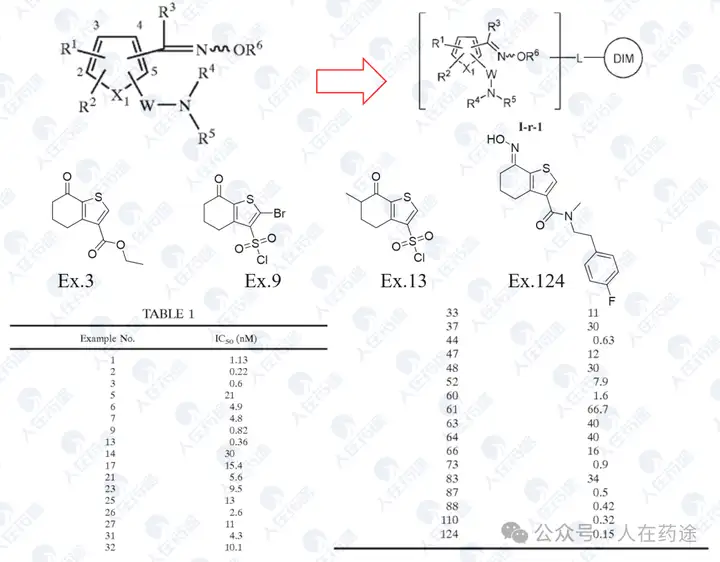
US20050227959A1, Eisai
Excellent inhibitory activity, but the structure is relatively simple.
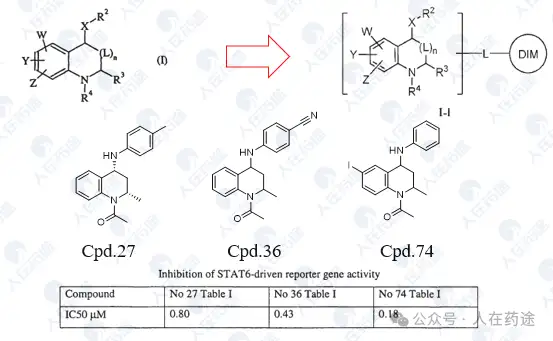
WO2007148711A1, Institute of Medicinal Molecular Design
High STAT6 inhibitory activity, but there is still NF-κB inhibitory activity.
3. STAT6 phosphorylation inhibitors
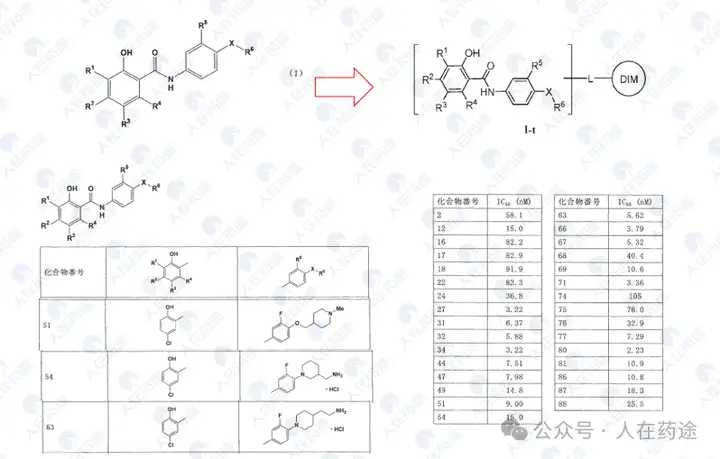
WO2001083517A1, Taisho Pharmaceutical/Tularik (Amgen)
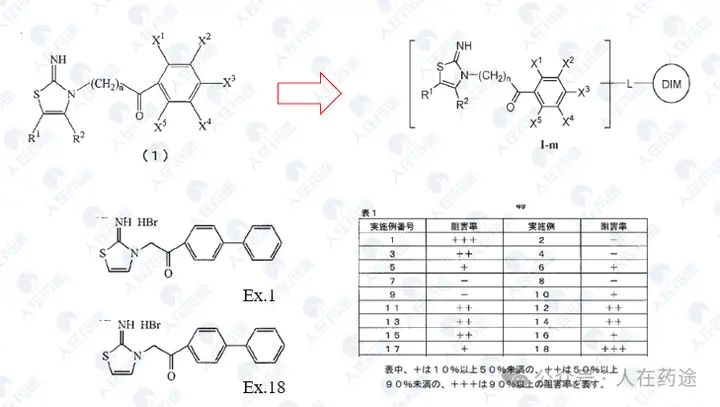
The key source patent for phosphopeptide STAT6 inhibitors. Although the activity is average (++: IC50 < 100 μM, but the data is questionable), the selectivity may also be average (the patent claims it as a STAT4/6 inhibitor but does not provide STAT4 inhibitory activity), yet it provides the original scaffold crucial for structural modification.
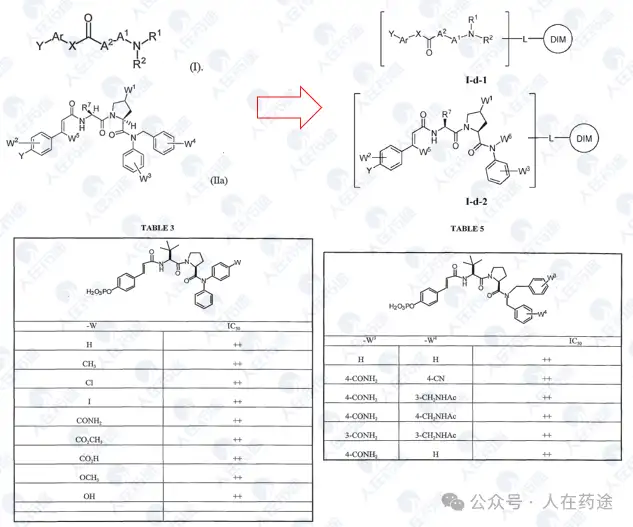
WO2002038107A2, Tularik (Amgen)
A derivative patent of the previous patent, with average activity (IC50 < 50 μM).
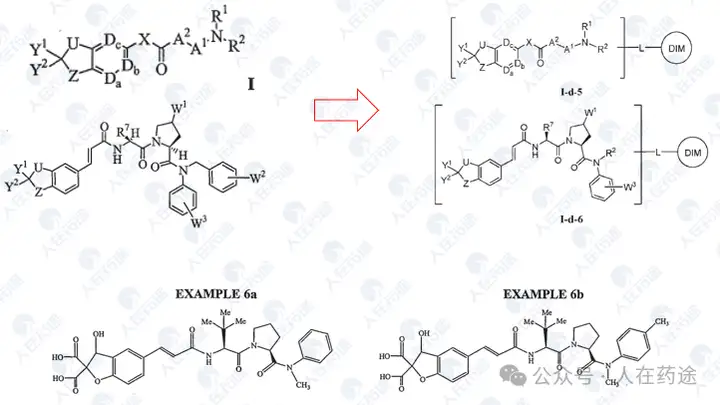
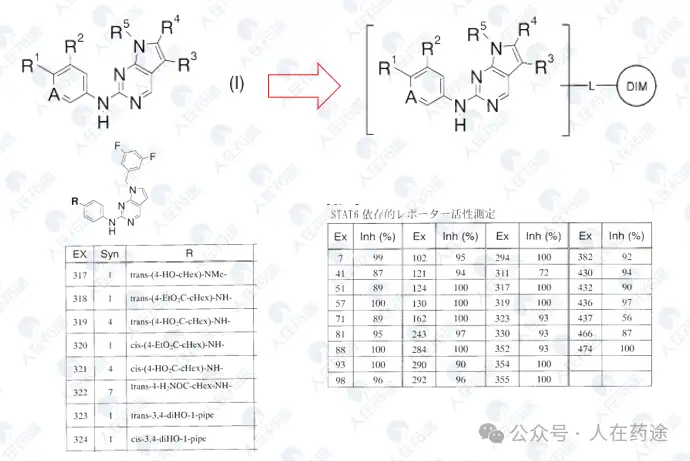
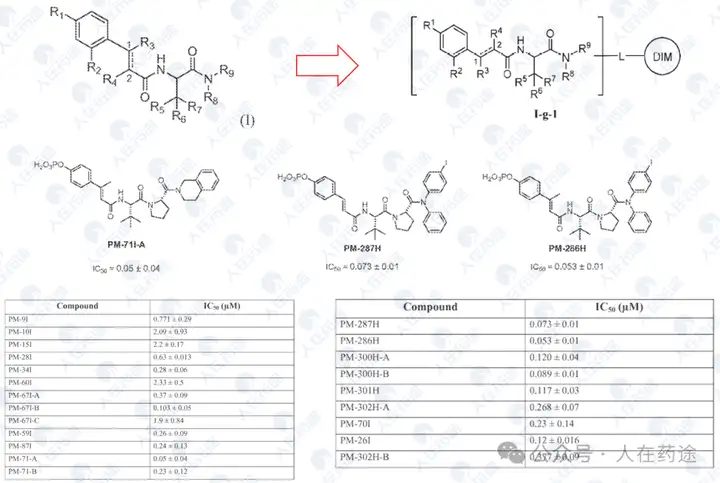
WO2014182928A2, University of Texas/Baylor College of Medicine
The activity of this patent has been greatly enhanced, with most compounds achieving STAT6 inhibitory activity of IC50 < 1 μM. Of course, this may also be primarily influenced by the screening system; for example, the PM-287H in the patent actually comes from Table 3 of WO2001083517A1.
Some cyclization strategies in this patent also form the basis for further optimization.
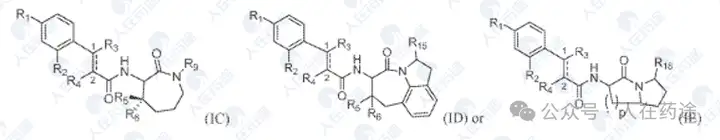
Massachusetts General Hospital, Bioorganic & Medicinal Chemistry, 2012, 20(2): 750-758.
The positive control 7Z in the patent comes from the aforementioned Eisai patent US20050227959A1, and the representative compound (R)-84 exhibits good STAT6 and its phosphorylation inhibitory activity, while 7Z has no effect on phosphorylation. It also shows high inhibitory activity and inhibition rates against inflammatory factors.
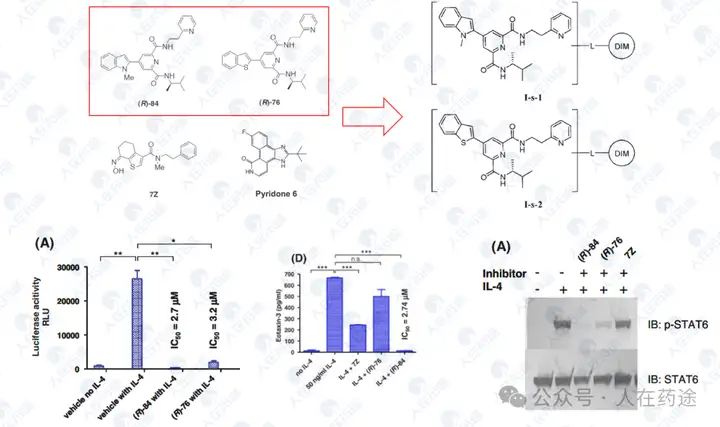
WO2002053550A1, Pola Chemical Industries
Good phosphorylation inhibition rate, but lacks specific inhibition activity data.
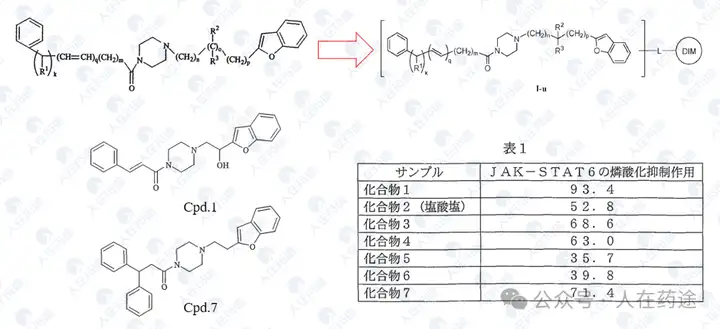
University of Texas/Baylor College of Medicine, J. Med. Chem. 2015, 58, 8970−8984
This article is actually a prodrug compound of patent WO2014182928A2, where Cpd.17 is the phosphate ester prodrug of M-287H, and many phosphate ester prodrug analogs Cpd.28-32 exhibit excellent phosphorylation inhibitory activity.
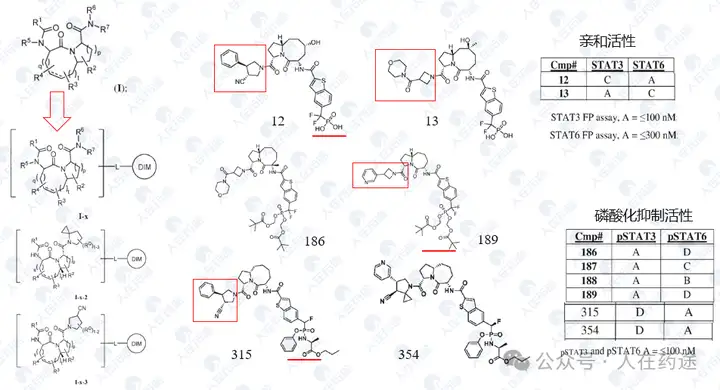
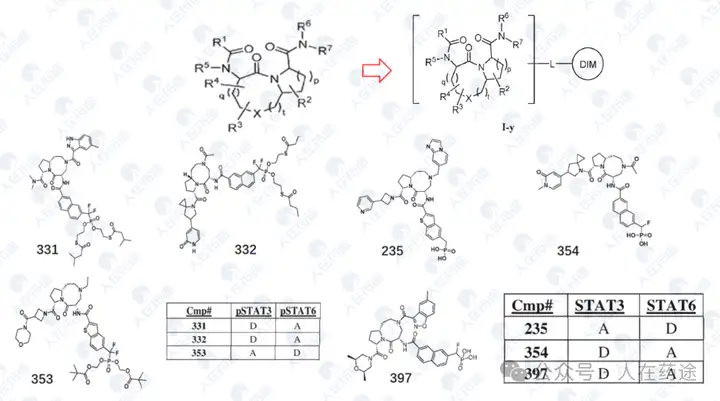
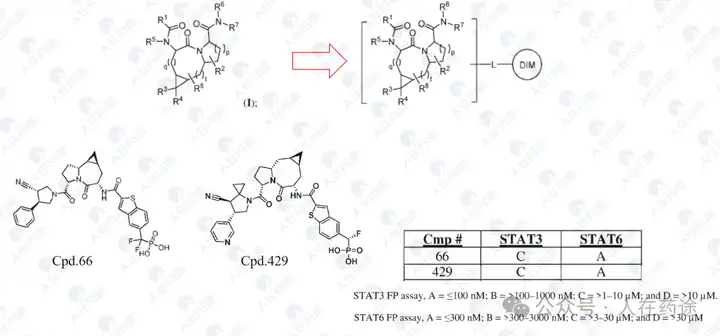
WO2023133336A1, Recludix Pharma
WO2023164680, Recludix Pharma
WO2023192960A1, Recludix Pharma
Conclusion
Due to the extensive content, this article's introduction is relatively brief. Kymera has listed 26 patents/literature related to STAT6 inhibitors in the patent. This article supplements it to 28 patents. Of course, some of these patents have low reference value, while others have high reference value, such as the most studied type, phosphopeptide inhibitors, which are likely the potential warhead of KT-621 (for a detailed introduction to phosphopeptide inhibitors, interested readers can refer to another article).
This analysis of the article ends here. As for the general structure of KT-621, stay tuned for the next breakdown.
As for other developments in this field, stay tuned for the next installment.

没有评论:
发表评论