The significance of the new E3 for PROTAC and molecular glue
As we all know, "Although the human genome encodes about 600 E3 ligases, only a few can be used for TPD so far, and only CRBN and VHL have entered the clinical trial stage." This sentence has always been a commonplace in the field of TPD. a topic. The development of new E3 ligase is one of the biggest bottlenecks in the current TPD field and a major blue ocean for future TPD development.
So, what is the significance of developing new E3 ligases?
For PROTAC:
1. Novel tissue-specific E3 ligase may reduce the on-target toxicity of PROTAC. The function of PROTAC relies on recruiting the expression of E3 ligase in the corresponding cells. Low-level expression of E3 ligase in unnecessary cells will minimize PROTAC activity and thus reduce side effects. An obvious example is Bcl-xL PROTAC, which takes advantage of the poor expression of VHL in platelets and successfully overcomes the platelet side effects caused by the original small molecule inhibitors.
2. The new E3 ligase may also avoid acquired resistance caused by genomic changes in the E3 ligase site.
3. Additional E3 ligases may target more challenging POIs. A large-scale degradation screen of the human kinome revealed that the degradation target space varies among E3 ligases. This variation in targeting specific targets by different E3 ligases may be due to unique PPIs between the E3 ligase and the target. Therefore, additional E3 ligases can expand PROTAC to more new targets. Of course, different E3 ligases will also lead to huge differences in selectivity. The previously mentioned SMARCA2 PROTAC is a good example .
Of course, the above three points naturally also apply to molecular glue degraders.
In addition, for molecular glues, the development of molecular glues based on CRBN is currently the mainstream, but the potential of CRBN is limited after all. The previous article also mentioned the current four-step strategy for the development of molecular glues. Evaluating the development potential of new E3 ligases and developing highly active E3 ligands based on this is also an important direction for the future layout of many TPD companies.
Some new developments in E3 development
In the field of PROTAC, some companies have also begun to report their preliminary progress in exploring new E3 ligases, but not many.
For example, Kymera, the leading TPD company, introduced its new progress in exploring tissue-specific E3 ligases in 2022, and designed STAT3 PROTAC for evaluation.
Vividion Therapeutics, a star company in covalent drugs, also introduced its covalent ligand-designed PROTAC developed for a new E3 ligase at AACR2020, which can achieve highly differentiated protein degradation of a wide range of targets.
Of course, it is a pity that they have no relevant documents/patents disclosed so far, so further evaluation cannot be made.
In recent years, a series of PROTAC-related documents/patents targeting new E3 ligases and ligands have been reported. However, most of these have poor degradation activity and can only be regarded as proof-of-concept and are still far from practical use.
However, the field of molecular glues is ahead of PROTAC in the exploration of new E3 ligases. The previously reported target-driven design of molecular glues (or monovalent degradation agents) based on "degradation tails", performance Excellent degradation activity across multiple targets. Of course, E3 choices are mainly limited to DCAF11 and DCAF16.
Although most of the reports in this area are BRD4 degraders (after all, BRD4 is really easily degraded). But the emergence of highly active and selective SMARCA2 molecular glue also gives people a bright feeling.
While sorting out the information, I also saw that Genentech reported a new molecular glue that relies on the E3 ligase FBXO22 at this year's AACR. Although it hides the structure of the final selective lead, from this result, we can roughly guess that the SMARCA2 molecular glue mentioned in the patent WO2023018648A1 above is likely to have this mechanism, not the DCAF16 I previously guessed (awkward) , guessed wrong again).
Of course, there is also the big oolong that came out of the previous patent about Amphista: I thought it was 26S, but it was actually DCAF16 .
Incidentally, 26S proteasomal degraders that do not rely on E3 ligases do have potential. An article published by the University of California on Biorxiv earlier this year confirmed this, which they called ByeTAC ( doi: 10.1101/2024.01.20.576376. ).
Choose the right E3
Assessing the tissue distribution of E3 and the PPI potential between E3 and POI seems to be an important direction in judging the development potential of new E3 ligases.
As mentioned in the previous summary: CRBN has a strong, feature-rich surface that can grasp a variety of new substrates and has high potential to form new PPIs; the shallow pocket of VHL requires large ligands and is relatively flat around the pocket. PPI potential is low.
Enriched protein surfaces not only lead to more PPI potential, but also make it easier to screen and discover high-affinity ligands. After all, only a glutarimide fragment is needed to bind to CRBN; VHL requires a peptoid fragment. Complex E3 ligands can lead to many potential problems. For example, VHL-based PROTACs are difficult to make into oral formulations.
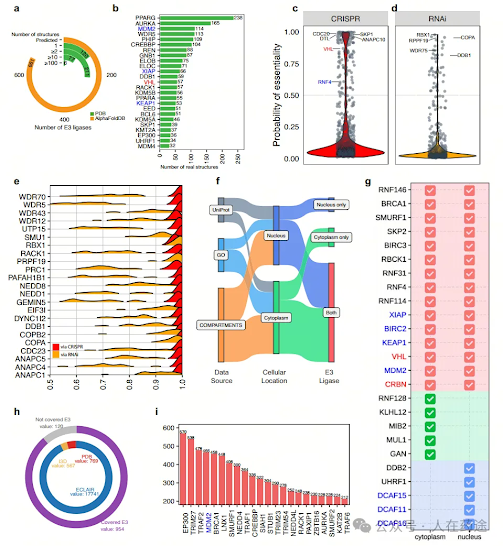
Of course, E3's organizational distribution and PPI potential are the core technologies that technology companies in the TPD field currently rely on most. There is currently very little information available through public channels. However, last year Indiana University published an article in Nature Communication, introducing the largest E3 database currently built based on biostatistics: E3Atlas ((https://hanlaboratory.com/E3Atlas/).
For example, they calculated the expression profiles of all E3 ligases in tumors.
As for other developments in this field, stay tuned for the next installment.
you can comment at bottom of the article or contact the author (https://www.linkedin.com/in/haixiang-pei-1a40b82b0/) on LinkedIn


















没有评论:
发表评论