Information
On October 28, 2024, Monte Rosa Therapeutics announced a global exclusive development and commercialization licensing agreement with Novartis to advance VAV1 MGD, including MRT-6160. MRT-6160 is currently undergoing a Phase 1 single ascending dose (SAD)/multiple ascending dose (MAD) study in healthy volunteers for immune-mediated diseases. Under the terms of the agreement, Novartis will receive global exclusive rights for the development, manufacturing, and commercialization of MRT-6160 and other VAV1 MGDs, and will be responsible for all clinical development and commercialization starting from Phase 2 clinical studies. Monte Rosa remains responsible for completing the ongoing Phase 1 clinical study of MRT-6160.
MRT-6160 is an effective, highly selective, orally bioavailable investigational VAV1 degrader, VAV1 is a key signaling protein downstream of T cell and B cell receptors. Preclinical studies have shown that VAV1 is deeply degraded, leading to a significant reduction in cytokines associated with immune-mediated conditions, with no detectable impact on other proteins. MRT-6160 has shown promising activity in various preclinical models of immune-mediated diseases.
According to the terms of the agreement, Novartis agrees to pay Monte Rosa an upfront payment of $150 million. Starting from the second phase of research, Monte Rosa is eligible to receive up to $2.1 billion in development, regulatory, and sales milestones, as well as tiered royalties on net sales outside the United States. Monte Rosa will co-fund any Phase 3 clinical development and will share any profits and losses related to the manufacturing and commercialization of MRT-6160 in the United States.
The Phase 1 SAD/MAD study of MRT-6160 is currently ongoing, with clinical data expected to be available in the first quarter of 2025.
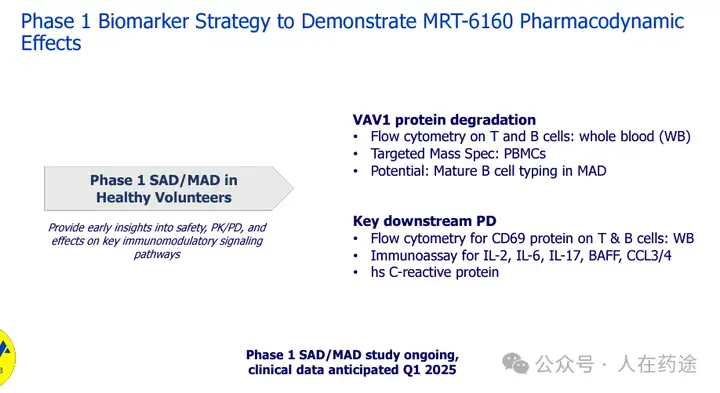
Relevant background information on VAV1 molecular glue has been previously introduced. This year's summary report on the molecular glue industry has also briefly introduced this. However, there has not been a detailed report on this, so here is a summary and supplement.
Background on the biology of VAV1
As early as August of this year, Monte Rosa published a report in Trends in Immunology regarding the biological background of VAV1 (DOI: 10.1016/j.it.2024.06.004), indicating that the transcription factor VAV1, as an "undruggable" protein, is a key positive regulator of T cell receptor (TCR) activation and cytokine production in primary human CD4+ and CD8+ T cells; data indicate that the loss/inhibition of VAV1 regulates autoimmunity and inflammation; and promising preclinical data from T and T/B cell-mediated arthritis and colitis disease models show the effectiveness of selectively targeting VAV1 through protein degradation.

VAV1 plays a key role in antigen receptor signal transduction
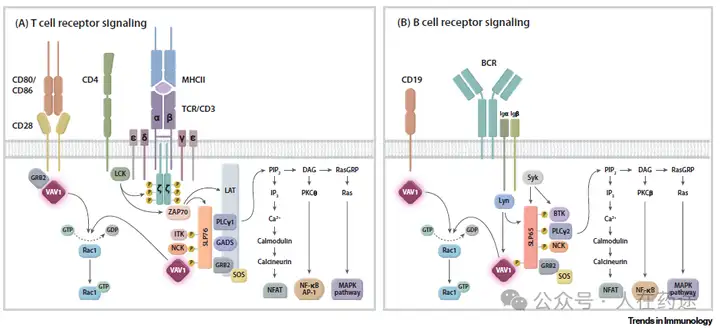
The core guanine nucleotide exchange factor (GEF) dependent function of VAV1
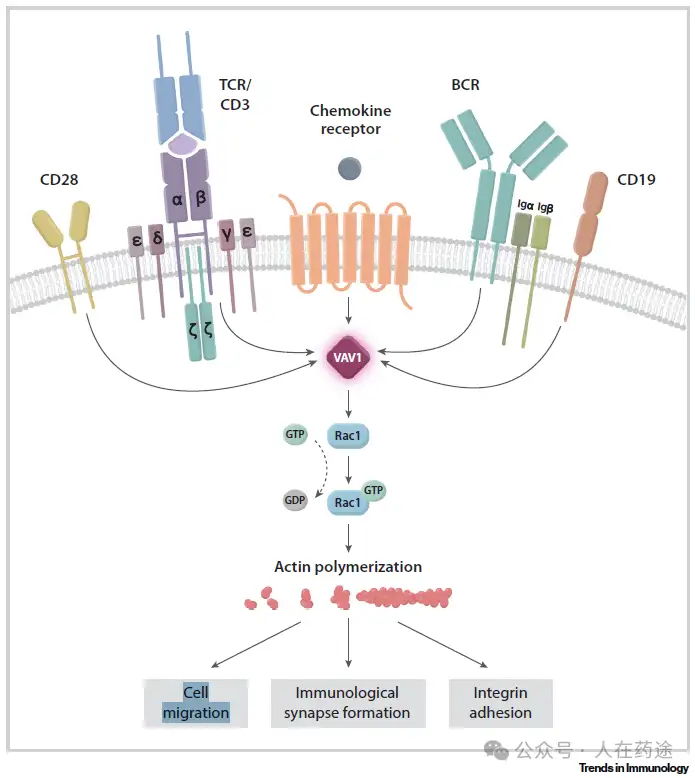
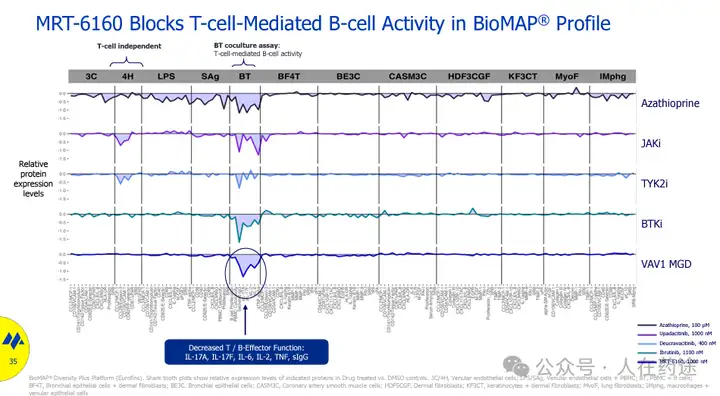
VAV1 is a validated key scaffold protein and signaling molecule downstream of T cell and B cell receptors. VAV1 knockout can block T cell-mediated B cell activity (IL-17A, IL-17F, IL-6, IL-2, TNF, sIgG).
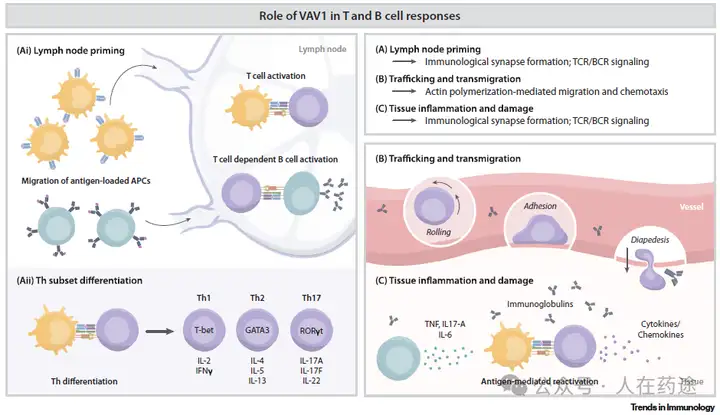
The commercial value of VAV1 molecular glue
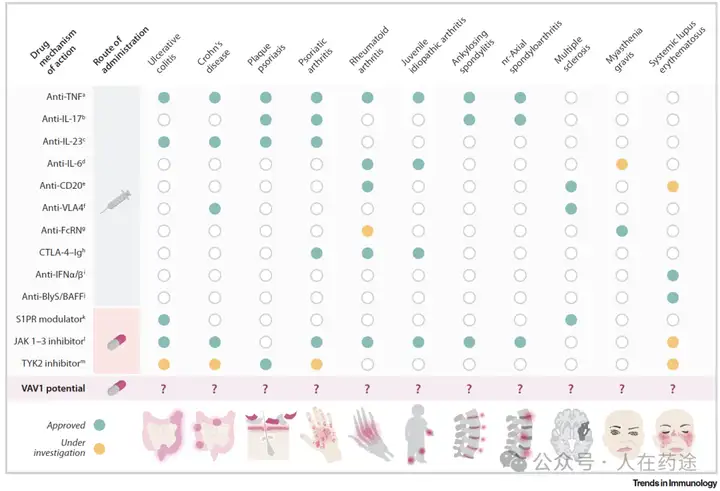
In recent years, the emergence of targeted therapies has brought breakthroughs in the treatment of autoimmune diseases and chronic inflammatory diseases, such as inflammatory bowel disease, rheumatoid arthritis, ankylosing spondylitis, and psoriasis, but there are still many unmet clinical needs. Currently, treatment strategies for autoimmune diseases and chronic inflammatory diseases mainly include biologics and oral small molecule drugs; however, issues such as treatment failure, poor drug tolerance, dependence on glucocorticoids, and lack of targeted therapies for certain diseases remain unresolved.
Existing treatment options, such as biologics, are effective in treating certain diseases, but they are often injectable and have issues such as immunogenicity. Oral small molecule drugs, while easy to administer, are limited in their scope of action because their targets are usually located intracellularly. Additionally, existing targeted therapeutic drugs often only inhibit a single protein or signaling pathway, leading to suboptimal efficacy. Therefore, developing more novel drugs targeting autoimmune diseases and chronic inflammatory diseases, especially those that can target multiple important signaling pathways and have safety and efficacy, remains an important direction for future research.
Compared to the shortcomings of the aforementioned therapies, the advantages of VAV1 molecular glue are mainly reflected in the following aspects:
- Strong targeting: VAV1 is primarily expressed in hematopoietic cells, including T cells and B cells, so molecular glue targeting VAV1 can more effectively suppress immune responses and reduce damage to other organs and tissues.
- Multiple mechanisms of action: VAV1, as a bifunctional protein, has both GEF activity and scaffold protein function. VAV1 molecular glue can simultaneously inhibit its GEF activity and scaffold protein function by degrading VAV1 protein, thereby blocking various important signaling pathways in T cells and B cells, more comprehensively suppressing immune responses.
- High efficacy: Studies have shown that VAV1 molecular glue exhibits good therapeutic effects in various animal models of autoimmune diseases, such as arthritis and colitis models, where VAV1 molecular glue can significantly reduce inflammation and improve tissue damage.
In summary, VAV1 molecular glue, as a novel targeted therapeutic drug, has three major advantages: strong targeting, multiple mechanisms of action, and high efficacy, and is expected to become a new hope for the treatment of autoimmune diseases and chronic inflammatory diseases.
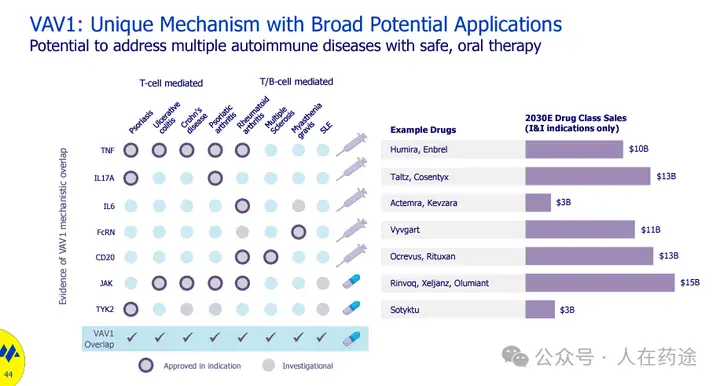
Monte Rosa also listed the relevant mechanisms and drug development trends of existing autoimmune therapies in the article, VAV1 molecular glue has a unique mechanism and broad potential applications, which can address various autoimmune diseases through safe oral treatment.
Experimental research has also shown that the VAV1 molecular glue has a more selective and more efficient blocking activity for T cell-mediated B cell activation.
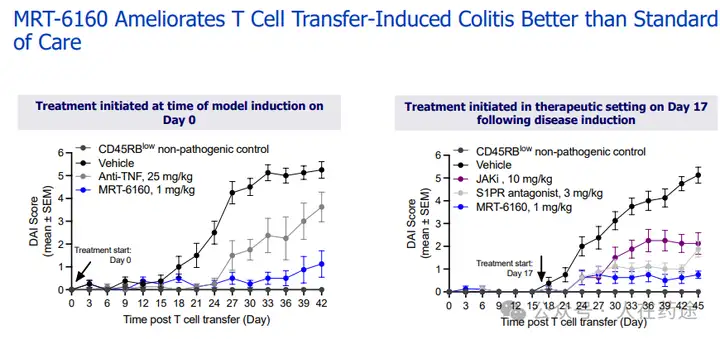
As for the in vivo efficacy of VAV1:
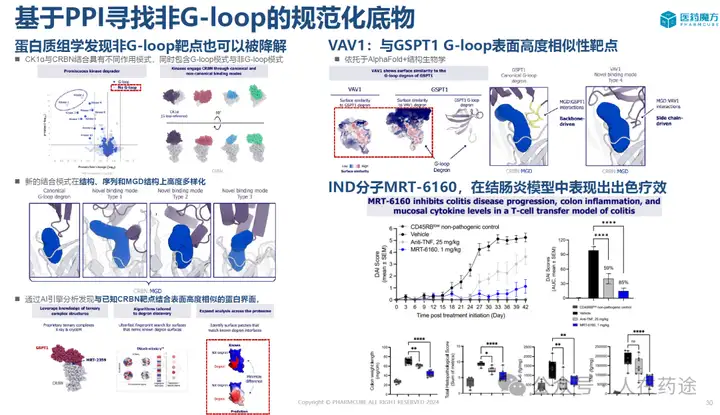
MRT-6160 improves T cell transfer-induced colitis better than standard therapy.
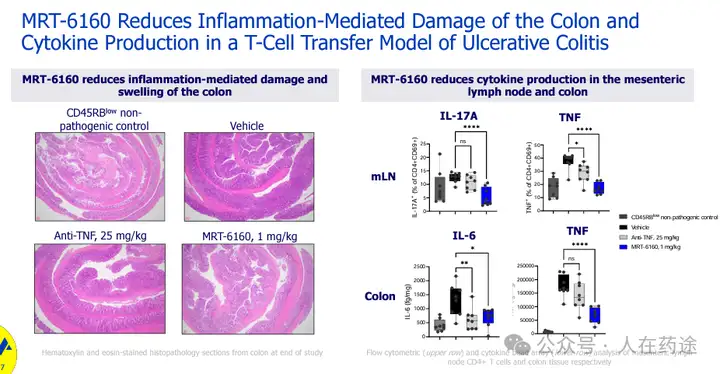
MRT-6160 reduces inflammation-mediated colonic damage and cytokine production in a model of T cell transfer in ulcerative colitis.
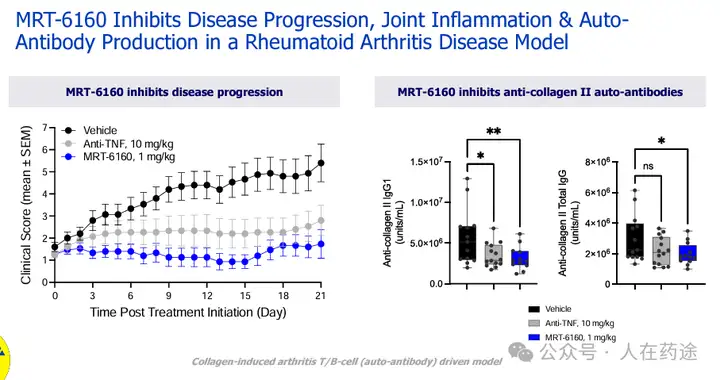
MRT-6160 inhibits disease progression, joint inflammation, and autoantibody production in a model of rheumatoid arthritis, also outperforming standard therapy.
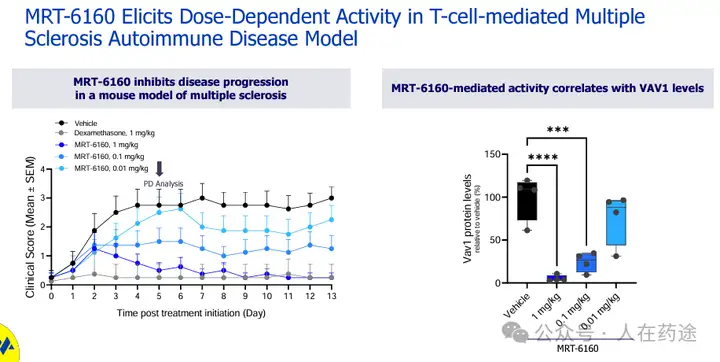
MRT-6160 induces dose-dependent activity in a model of T cell-mediated multiple sclerosis autoimmune disease.
Monte Rosa therefore has high expectations for this project, believing it to be a potential oral alternative therapy for various autoimmune diseases, likely to have a strong impact on the current billion-dollar autoimmune drug market.
What does MRT-6160 look like?
At the most critical stage, what does the specific structure of Monte Rosa's VAV1 molecular glue MRT-6160 look like?
In fact, by combining publicly available poster information and patents (WO2024151547A1), it is easy to guess.
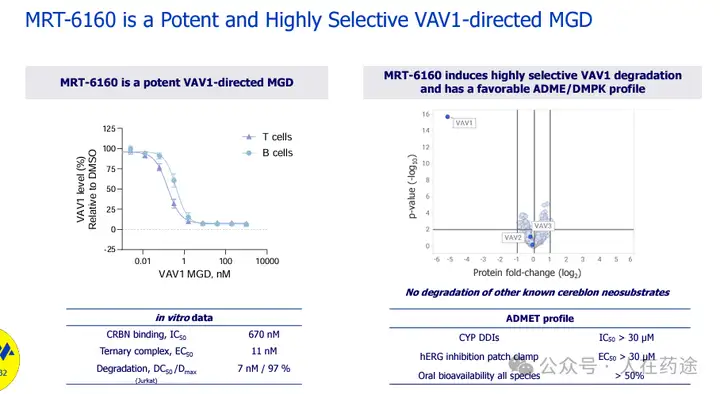
First, the degradation activity of MRT-6160: DC50 = 7 nM, Dmax = 97%.
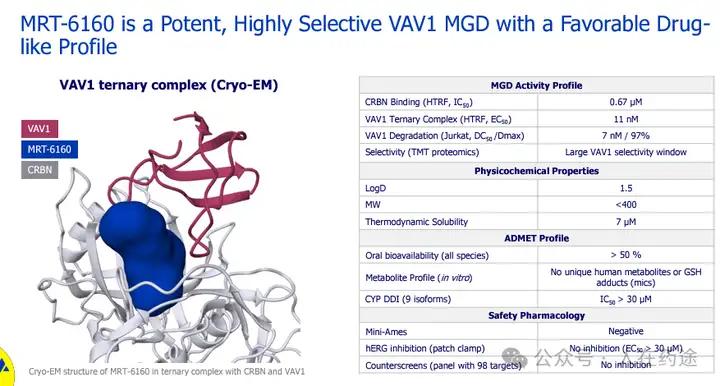
What is the situation in the patent? Here, I will lazily borrow content from previous reports; it is evident that through activity data, simple SAR analysis, and drugability assessment, Cpd.185 is very likely the specific structure of MRT-6160.
Epilogue
It was previously mentioned that VAV1 is a target with a high similarity to the G-loop surface of GSPT1. The discovery of VAV1 molecular glue relies on the QUEEN platform from Monte Rosa, developed based on AI and structural biology, and is a true AIDD drug.
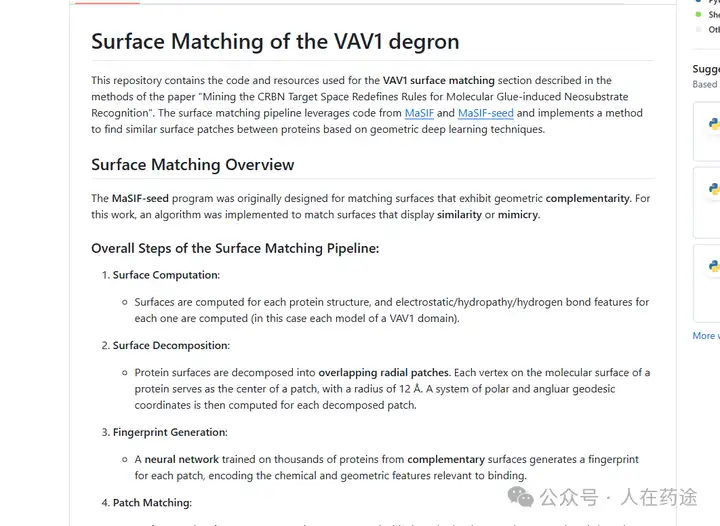
Even more astonishingly, the great benefactor has open-sourced this project on GitHub. Those interested can run it to help discover new VAV1 molecular glue.
The first attention to the VAV1 molecular glue began last July. The project, which has long been promising, has finally received preliminary confirmation through this deal with Novartis, which is quite delighting. I look forward to future clinical results further confirming this. The writing is rushed, and the content is a bit hurried, so please forgive any mistakes.
Welcome to forward, share, and make reasonable citations. When citing, please indicate the source prominently; if you need to reprint, please leave a message or send a message to the WeChat public account backend, and specify the public account name and ID. Original articles may not be reproduced without authorization, and violators will be held legally responsible.
Disclaimer: This article only represents personal views and does not represent the company's position.