Background
CAR-T cell therapy has become a promising cancer treatment strategy. Despite impressive clinical efficacy, the widespread application of current CAR-T cell therapy is severely limited by treatment-related toxicities (uncontrollable hyperactivity, which may lead to severe toxicity). At the same time, CAR-T cell exhaustion is a major limitation of its efficacy, especially in the application of CAR-T cells to solid tumors.
The ability to control the interaction between CAR-T cells and cancer cells using switch molecules allows for the regulation of T cell therapeutic functions while retaining antigen specificity. This titratable pharmacological modulation enables physicians to precisely control the timing, location, and dosage of T cell activity, thereby alleviating toxicity. It may also expand the scope of CAR-T therapy to solid tumors and indications beyond cancer treatment.
Compared to the lack of regulatory capability, the ability to regulate CAR expression in the presence of switch molecules has many advantages:
- 1. Targeted but non-tumor effects mediated by therapeutic immune cells expressing CAR may lead to toxicity, which can be reduced or eliminated by the switch.
- 2. CAR-mediated immune responses that are too strong can be reduced or eliminated by the switch.
- 3. Avoiding T cell dysfunction caused by chronic activation and overexpression of checkpoints can be achieved through cyclic CAR expression or titration of CAR expression.
Based on this concept, numerous titratable pharmacology-based CAR-T therapy "safety switch" control strategies have begun to emerge (Science. 2015 Oct 16;350(6258):aab4077; Proc Natl Acad Sci U S A. 2016 Jan 26;113(4):E450-8; Blood Cancer J. 2018 Aug 22;8(9):81; https://meiragtx.com/wp-content/uploads/2022/10/P399-RiboCAR-ESGCT-2022.pdf; Cancers (Basel). 2021 Sep 22;13(19):4741). These strategies are valuable research tools and models for future cell therapy.
Breakthrough technology: Molecular glue as a safety switch for CAR-T
In 2021, the Dana-Farber Cancer Institute collaborated with Massachusetts General Hospital to publish an article in Science Translational Medicine, introducing the use of molecular glue drugs to lenalidomide as a reversible switch for CAR-T therapy.
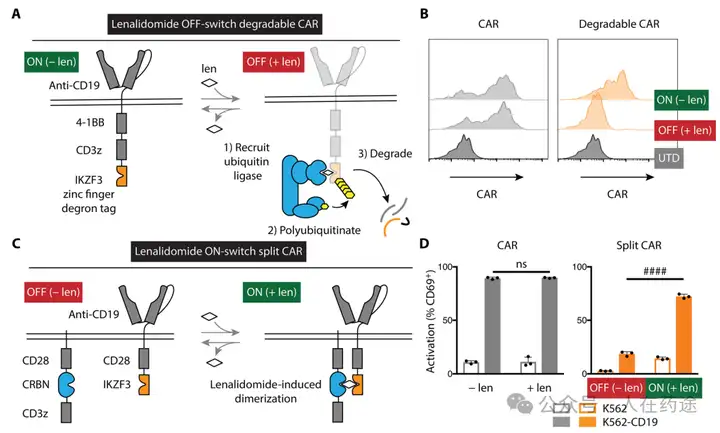
The article designed two switch modes: "OFF-switch" degradable CAR (the CAR contains a lenalidomide-dependent degradation tag. Upon the addition of lenalidomide, this tag is recognized and degraded by CRBN, leading to the inactivation of CAR-T cells) and "On-switch" split CAR (the CAR consists of two subunits, each containing a part of the CAR. These two subunits can bind through lenalidomide-induced dimerization interactions, thereby activating CAR-T cells), both demonstrating effective control of CAR-T activity.
Furthermore, through screening, a heterozygous degradation determinant (ZFP91-IKZF3) was discovered, which has a higher sensitivity to lenalidomide compared to the parental line. Introducing this degradation determinant into the aforementioned two "switch CARs" significantly enhances the regulatory activity of lenalidomide.
Mechanistically, the "OFF-switch" degradable CAR has certain advantages over the "On-switch" split CAR, such as a simpler structure, more flexible control, and potentially lower toxicity. Further in vitro and in vivo activity evaluations in the article are also based on the "OFF-switch" degradable CAR.
In vitro activity evaluations indicate that lenalidomide can effectively inhibit the cytotoxicity and cytokine secretion of CAR-T cells and control their proliferation. Meanwhile, after the withdrawal of lenalidomide, CAR-T cells can rapidly regain activity.
In vivo efficacy indicates that the design of the "OFF-switch" degradable CAR does not affect activity and can still significantly inhibit tumor growth. Lenalidomide can effectively suppress cytokine release from CAR-T cells and reduce their toxicity.
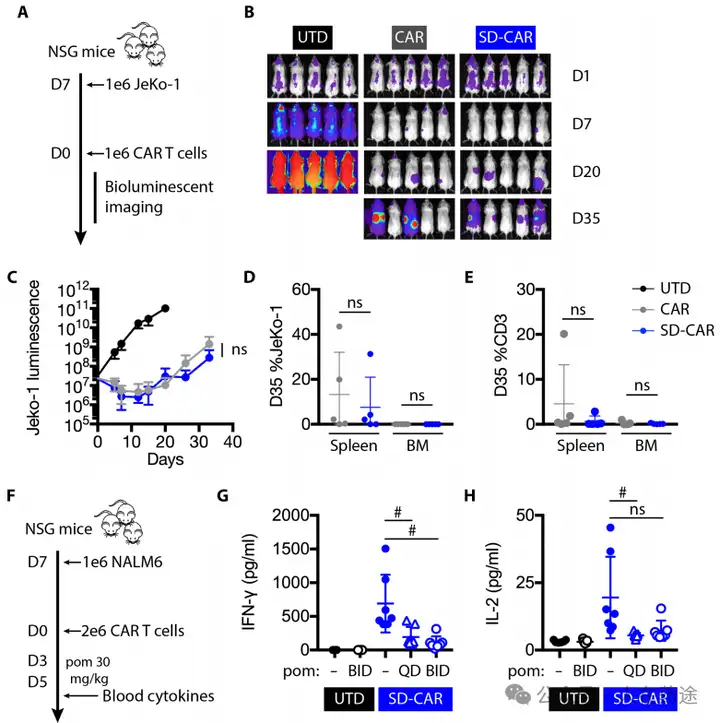
Compared to previous studies, the sensitivity and simplified structure of the lenalidomide-based "OFF-switch" degradable CAR may be the most effective strategy for flexibly controlling CAR-T therapy activity in the future. However, it also has certain limitations, such as insufficient sensitivity of the degradation tag and the lack of selectivity of lenalidomide, which may lead to potential side effects. This also points to further optimization directions for subsequent research.
Celgene's highly selective CAR-T reversible switch molecular glue
After discussing the article, it is natural to mention the patents.
Celgene publicly disclosed two related patents in 2023 and 2024 (WO2023015283A1, WO2024167999A1), which clearly inherit the research ideas from the aforementioned Science Translational Medicine article, introducing a class of high-activity, high-selectivity molecular glue drugs applied to "OFF-switch" degradable CARs.
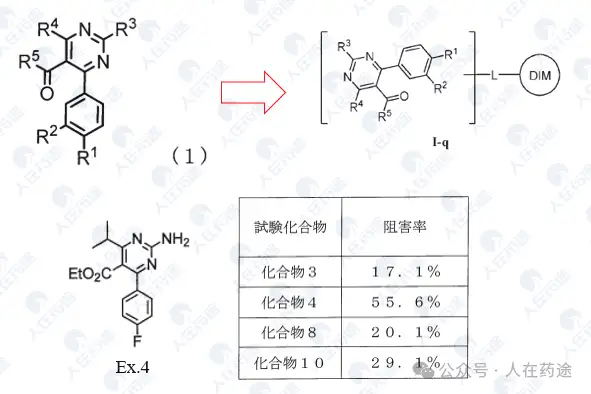
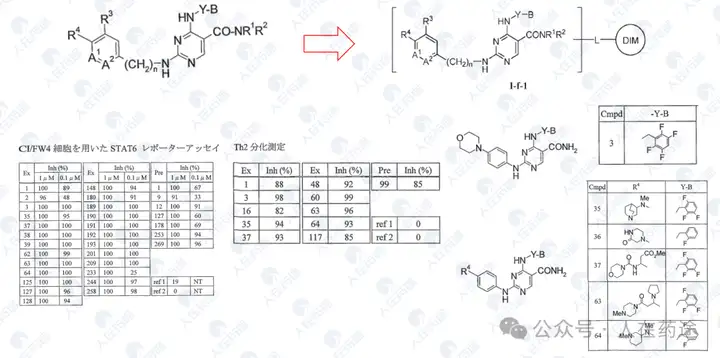
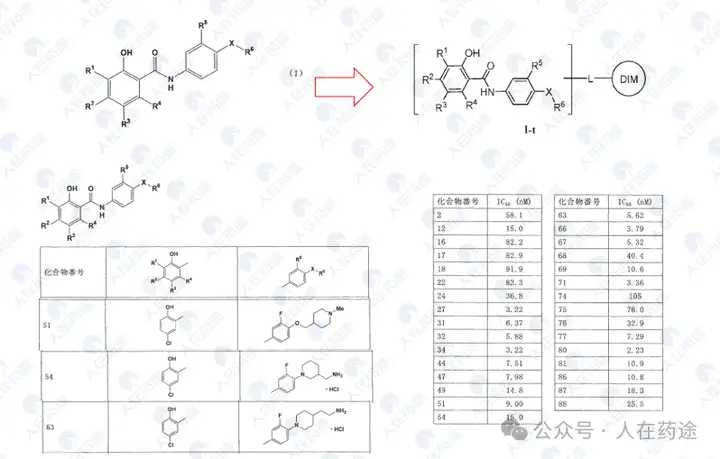
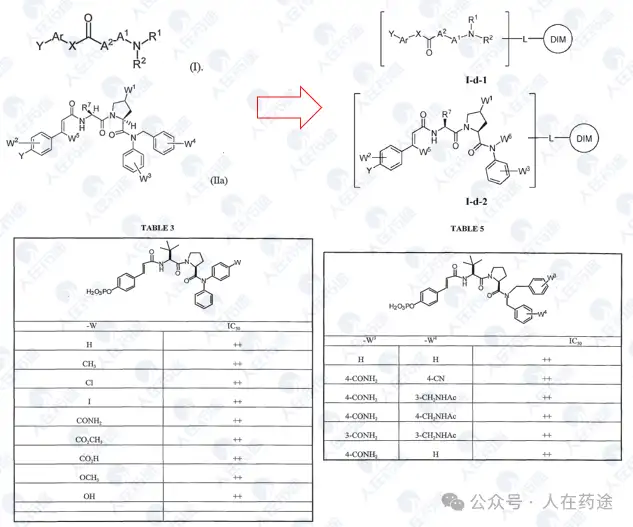
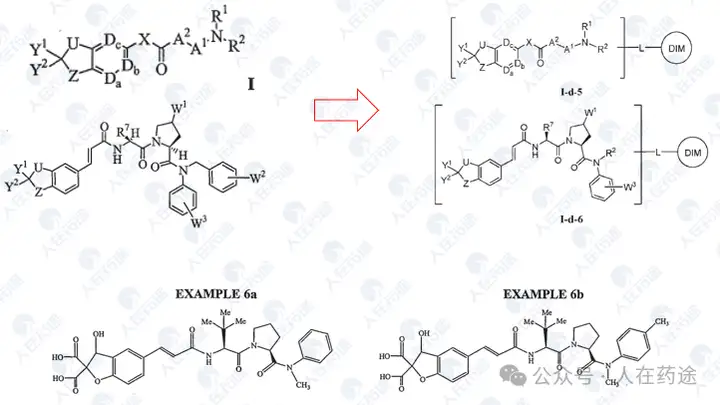
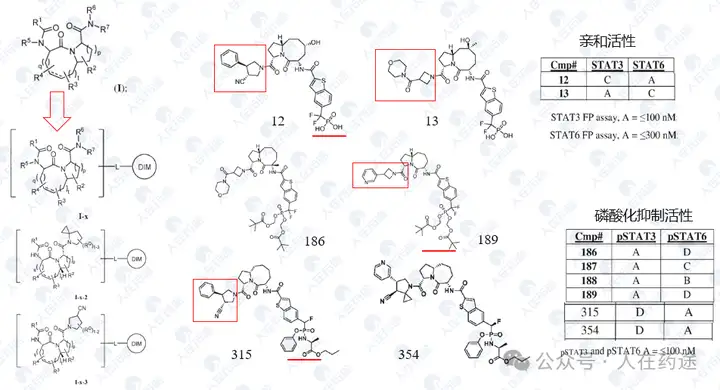
WO2023015283A1, the most critical patent. Unlike the aforementioned article's choice of IKZF3, Celgene's degradation target selected IKZF1.
The patent first designed a chimeric CD19 CAR containing a G-loop degradation determinant [Reference: Rational Design of Molecular Glue (3): Monte Rosa teaches you how to "train" CRBN], first verifying that the CAR marked with IKZF1 ZNF2 does not affect functionality. Secondly, Cpd.A was used to degrade the CAR, but Dmax was only 56%. Such low activity is clearly insufficient.
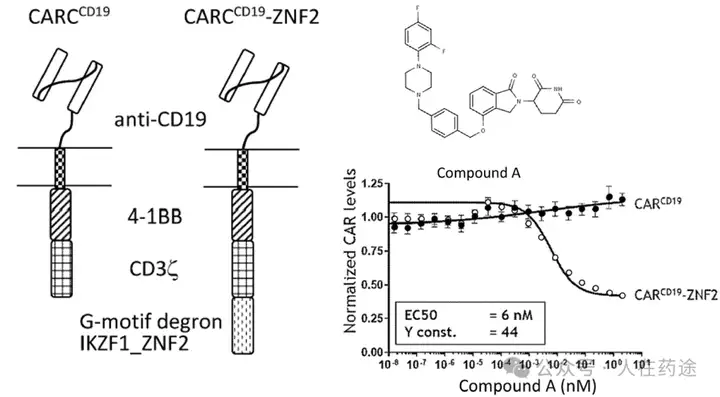
As in the article, the next step is to optimize the degradation determinant to respond more effectively to Cpd.A. The sequence containing motif ZNF2_3 (SEQ: QCNQCGAS) exhibited the highest impact rate on Cpd.A (Dmax = 99.1%) and specificity (inactivation after G6N glycine mutation).
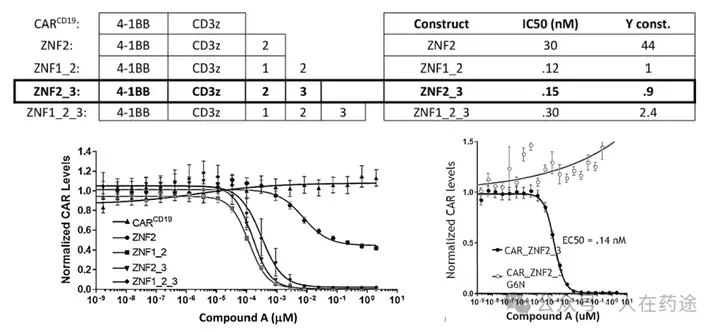
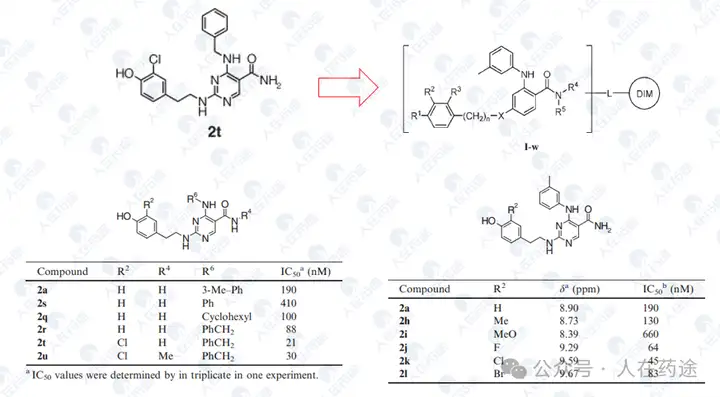
After optimizing the high-response motif, the next step is to address the selectivity issue. Celgene's method is to perform site-directed mutagenesis on the G-loop (SEQ: QCNQCGAS changed to FCNQCGAS), namely IKZF1 ZNF2_3 Q1F.
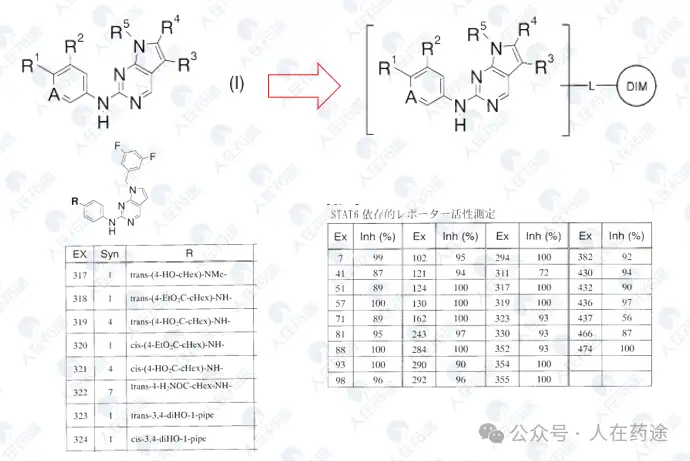
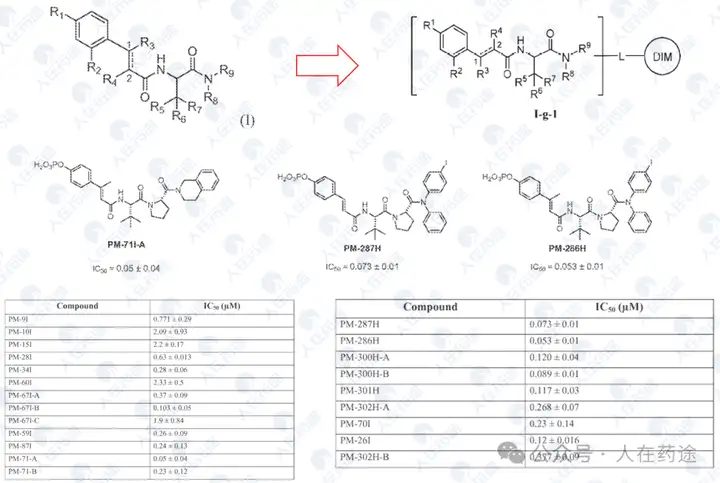
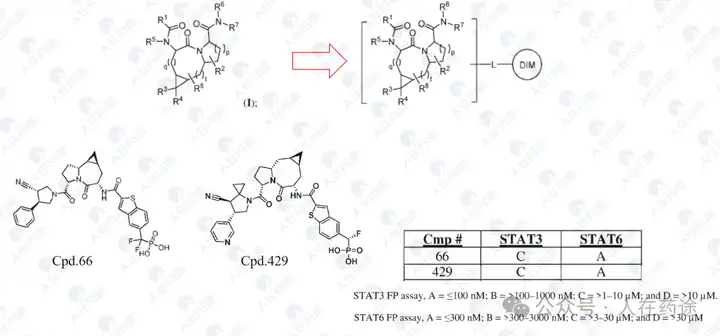
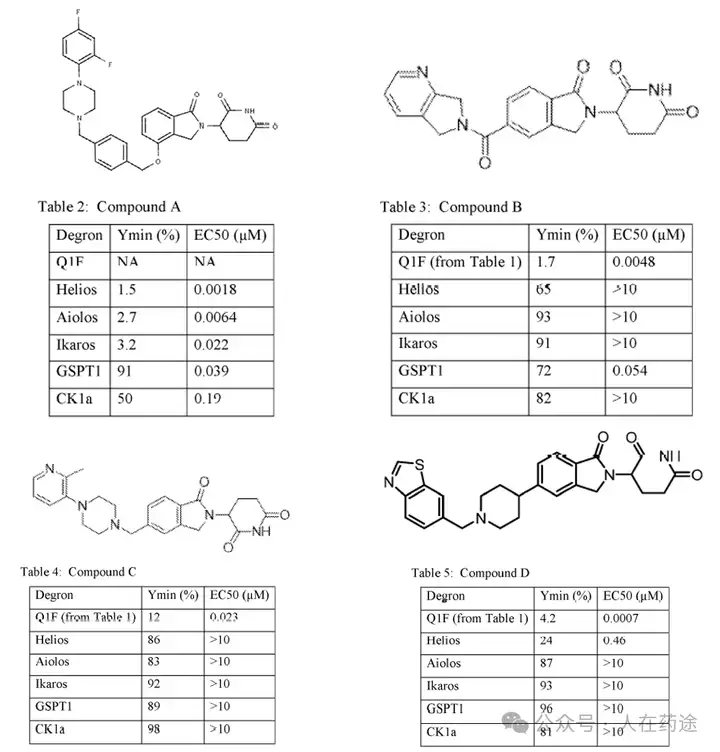
Compound screening and selectivity evaluation found that Cpd.A exhibited high activity against IKZF1/2/3 (Ikaros/Helios/Aiolos), CK1α, and GSPT1, but showed no activity against Q1F. Meanwhile, Cpd.B, C, and D all exhibited high activity against Q1F while showing certain selectivity against IKZF1/2/3, CK1α, and GSPT1. Notably, Cpd.D was the most optimal, showing high activity against Q1F (DC50 = 0.7 nM) while only slightly less selective against IKZF2 (DC50 = 460 nM). This also became the structural basis for further optimization in the future.
The next step is to evaluate the regulatory capability of selective specific molecular glue on "OFF-switch" CAR-T activity. To demonstrate the universality of this technology, Celgene constructed a new ROR1 CAR-T (IKZF1 ZNF2_3 Q1F).
Regarding the issue of CAR-T hyperactivity, Cpd.C can concentration-dependently regulate the production of pro-inflammatory cytokines, indicating that the effector function of CAR-T can be modulated through the titration of degradation of Q1F marked CAR by molecular glue.
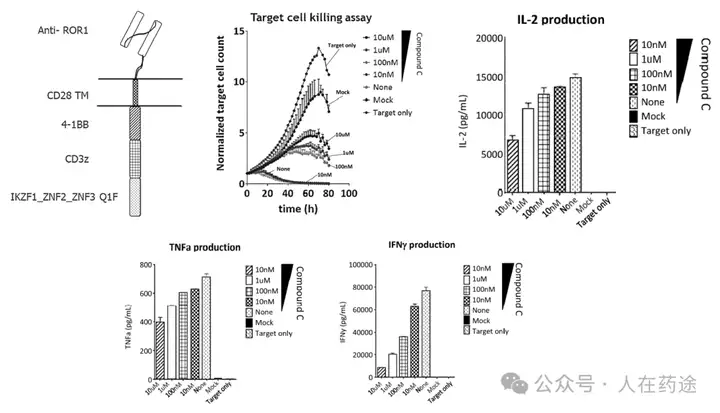
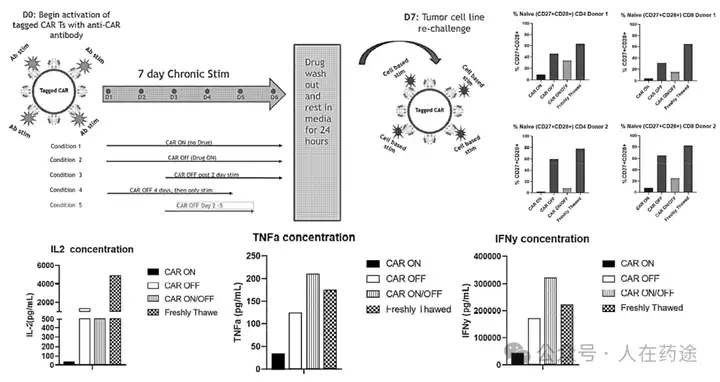
Regarding T cell exhaustion in CAR-T, the loss of cytokines such as IL-2, TNF-α, and IFN-γ in the lack of regulatory CAR-T (CAR ON) is a hallmark of T cell exhaustion. The addition of Cpd.D can provide CAR-T cells with a brief rest period, leading to less activation and maintaining a more naive population. This brief rest period also provides functional benefits for the production of pro-inflammatory cytokines. Meanwhile, timed and quantitative administration of Cpd.D (CAR ON/OFF) shows significant therapeutic benefits.
Further in vivo evaluations based on the aforementioned characteristics indicate that Cpd.D can significantly promote the in vivo degradation of CAR and respond quickly, with CAR expression essentially restored after 48 hours. Meanwhile, CAR degradation reduces the expansion and lytic function of CAR T cells marked with the degradation determinant, demonstrating the ability of CAR cycling to provide functional rest for CAR-T cells.
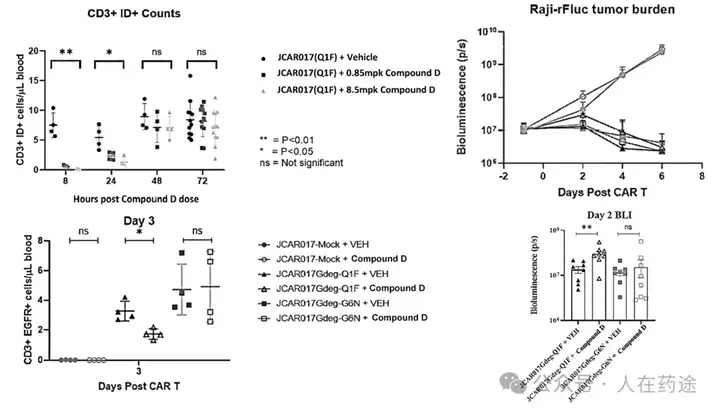
Cpd.D in this patent significantly demonstrates high activity and high selectivity in regulating CAR-T, consistent with the results from the aforementioned Science sub-journal, and addresses the issues of response rate and selectivity of lenalidomide. Cpd.D can significantly modulate CAR-T cell activity, avoiding treatment side effects and T cell exhaustion caused by hyperactivity, making it safer and more effective.
Of course, Cpd.D still has certain flaws, namely that its selectivity for IKZF2 is still insufficient.
This issue has been effectively addressed in Celgene's latest patent WO2024167999A1.
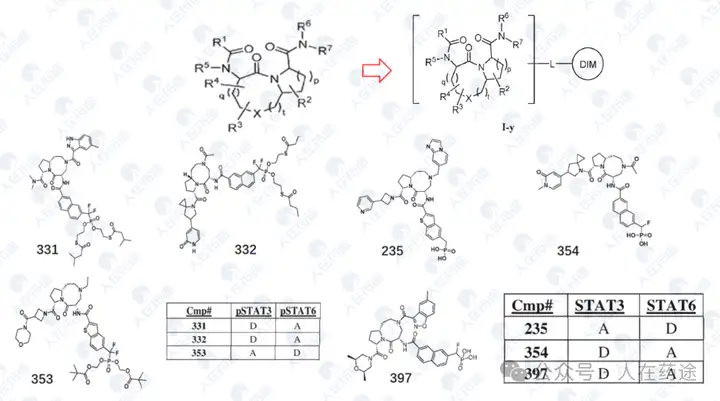
This patent further optimizes the Cpd.D structure. Firstly, the introduction of two fluorine atoms on the CRBN ligand significantly enhances selectivity (DC50 of IKZF2 > 10 μM), while having a minimal impact on Dmax and also improving activity (Ex.54 vs Cpd.D). Furthermore, attempts to substitute the key group on the left side resulted in numerous compounds with varying activity and selectivity, with some examples demonstrating excellent activity and selectivity, particularly Ex.59 (DC50 = 0.01 nM, Dmax).
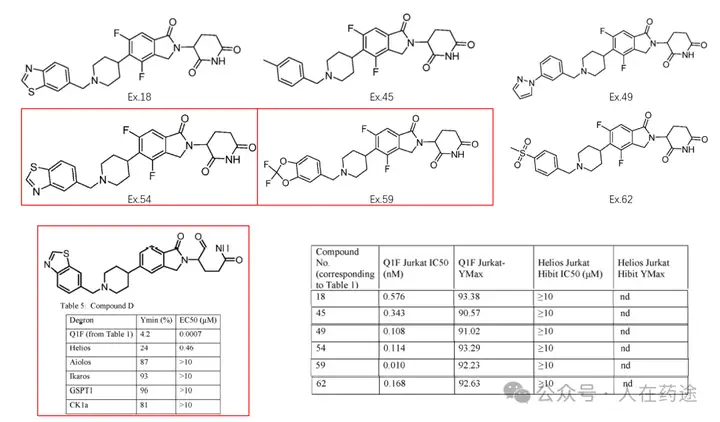
The patent also claims selectivity for IKZF1/2/3, CK1α, and GSPT1, but the data has not been presented; it is believed that it should not be inferior.
Afterword
In summary, Celgene/BMS has developed a set of molecular glue tool compounds that can effectively address the therapeutic side effects and T cell exhaustion associated with CAR-T therapy, demonstrating long-term application prospects.
It must be said that BMS's investment in the development of molecular glues, market positioning, and research depth is at the forefront of the industry.
To draw on previous examples, it can be observed that after acquiring Celgene, BMS has successively established technology platform collaborations with five biotech companies specializing in different research directions related to molecular glues.
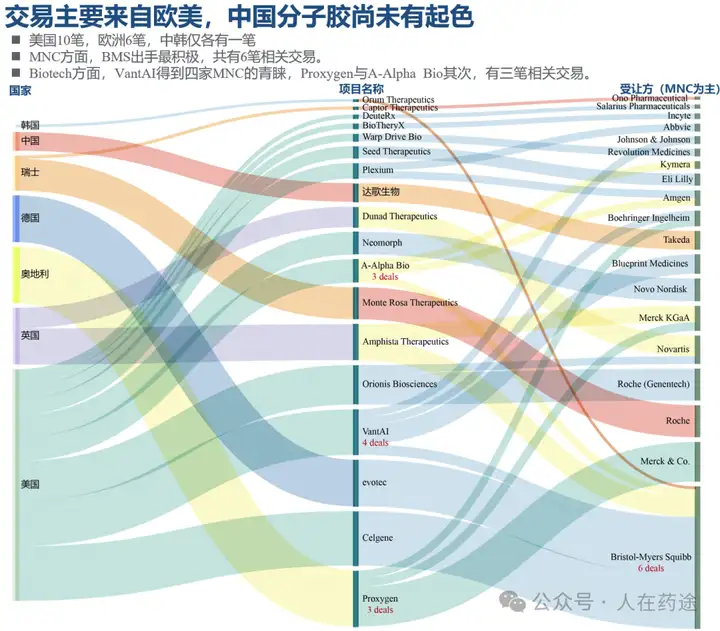
At its R&D day in 2023, BMS also demonstrated its long-term planning in the field of molecular adhesives.
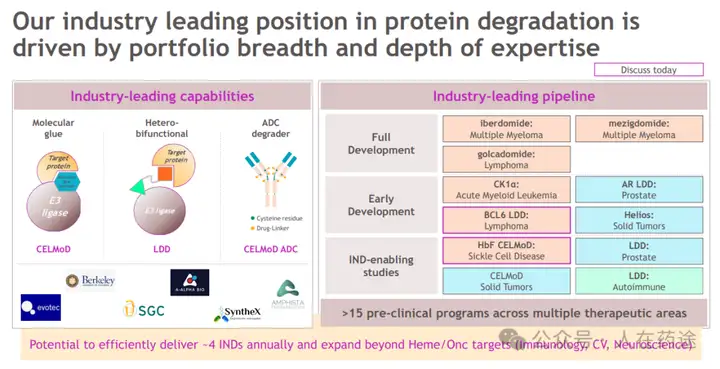
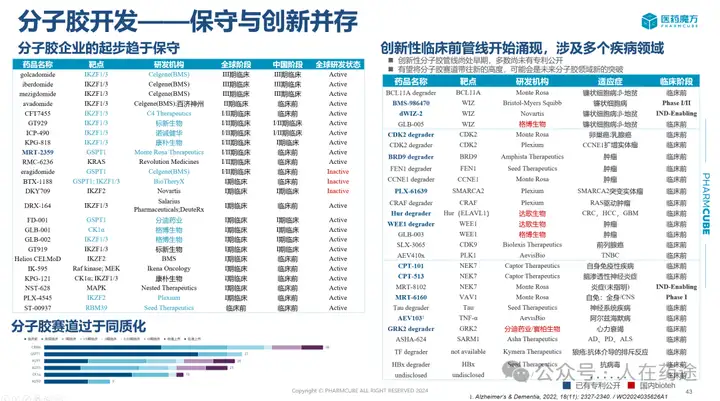
At the same time, in the current conservative environment of molecular adhesive development, BMS has proposed numerous innovations.
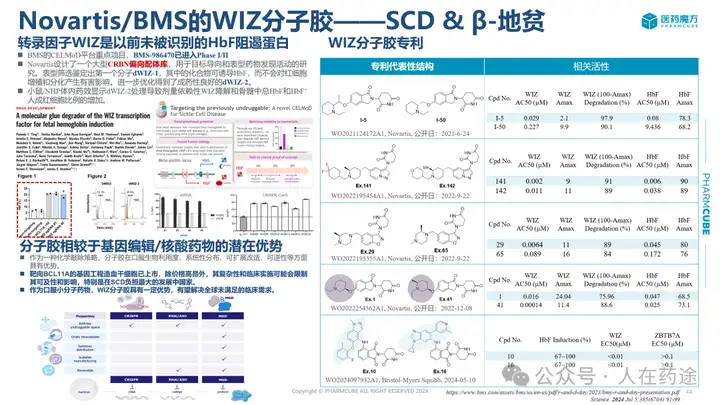
For example, the WIZ molecular glue mentioned earlier has now entered Phase I.
This time, a new idea for the application of molecular adhesives has also been presented. Although BMS was not the first to propose this concept, it has made groundbreaking improvements based on it, making it more valuable for application.
Looking forward to more highlights emerging in the field of molecular adhesives in the future.
We can also provide academic promotion, project research, patent information consulting and other related services. You can comment at bottom of the article or contact the author (https://www.linkedin.com/in/haixiang-pei-1a40b82b0/) on LinkedIn
As for other developments in this field, stay tuned for the next installment.